The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View mm content recommended for you
Tailoring first-line therapy to MRD status: The MASTER trial final analysis
Quadruplet induction therapy consisting of daratumumab, carfilzomib, lenalidomide, and dexamethasone has shown durable depth and duration of responses in patients with newly diagnosed multiple myeloma, including those treated with autologous stem cell transplantation (ASCT).1 However, the clinical benefit of continuous quadruplet therapy after transplant and its associated patient response has yet to be fully explored.1
In order to bridge this knowledge gap, Costa1 carried out the MASTER trial (NCT03224507), which aimed to determine the rate of measurable residual disease (MRD) negative responses with quadruplet induction therapy and a response-adapted consolidation. We summarize the final analysis presented at the European Hematology Association 2023 Hybrid Congress. For prior results and more information on the MASTER trial, check out our previous article here.
Study design
- For the complete study design, see our previous article on the MASTER trial here
- The eligibility criteria for patients were:
- Untreated newly diagnosed multiple myeloma with measurable disease
- Eastern Cooperative Oncology Group performance status 0–2
- Creatine clearance ≥40 mL/min
- No cardiopulmonary disease, concomitant or recent malignancy, or significant neuropathy before study enrolment
- MRD was assessed at each stage of treatment at a level of <10-5 using next-generation sequencing
- There was an enrichment of patients with high-risk cytogenetic abnormalities, defined as a gain or amplification of 1q, t(4;14), t(14;16), t(14;20), or del(17p)
Results
Efficacy
- A total of 123 patients were included in the trial. For the complete patient characteristics at baseline, visit the previous article here.
- Overall, 81% of patients achieved MRD negativity and 71% achieved the MRD-SURE status defined as MRD-free, treatment-free state and ceased therapy.
- The rates of overall negative MRD and sustained negative MRD after 12 months according to the number of high-risk cytogenetic abnormalities are shown in Figure 1.
- Overall rates were similar regardless of cytogenetic risk, although most patients in the ultra-high-risk subgroup only achieved MRD-negativity status after transplantation and/or consolidation therapy.
Figure 1. Rates of overall MRD and sustained MRD after 12 months according to the number of high-risk cytogenetic abnormalities*
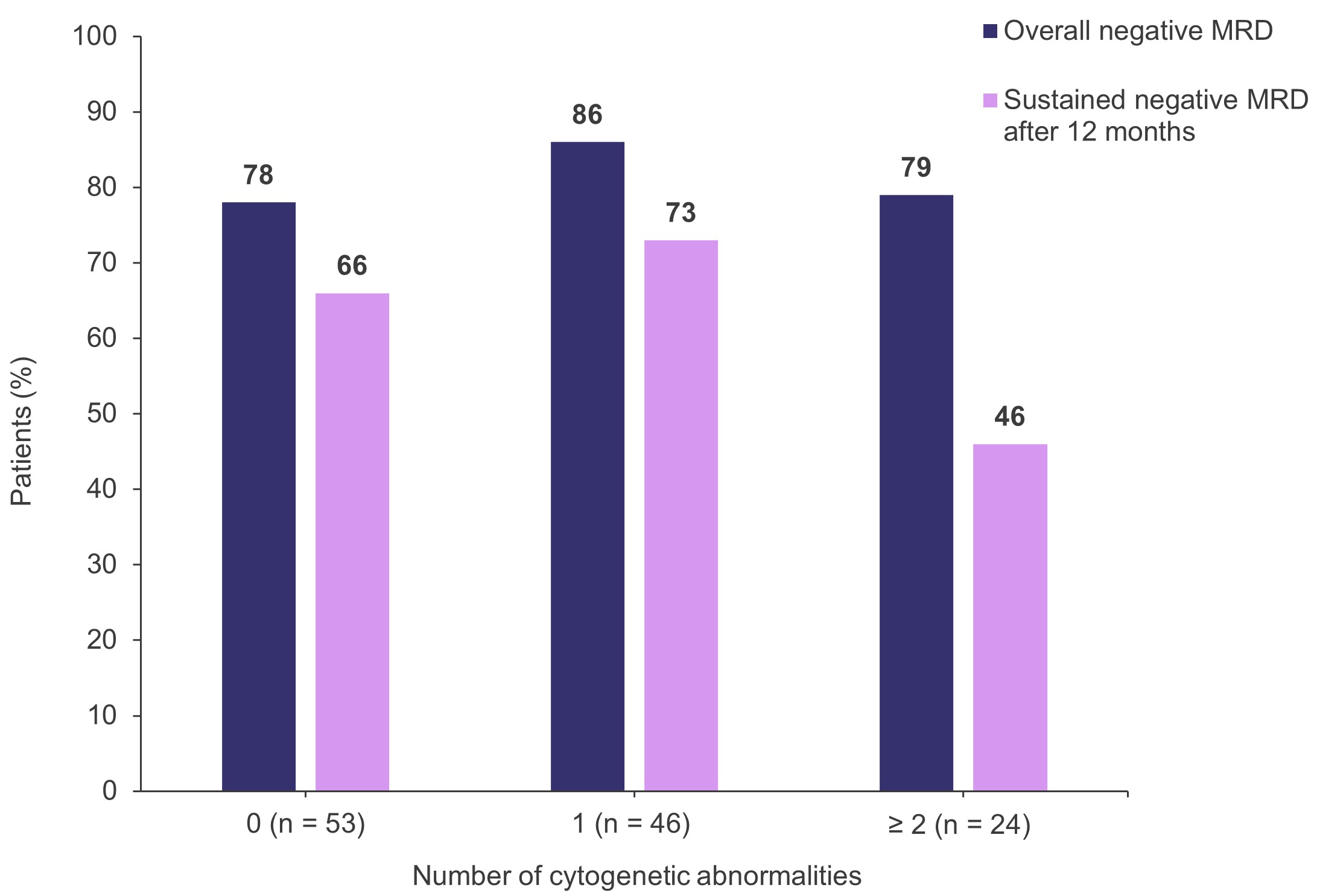
MRD, measurable residual disease.
*Adapted from Costa.1
- The rates of 3-year overall survival (OS) and 3-year progression-free survival (PFS) according to the number of high-risk cytogenetic abnormalities are shown in Figure 2.
- Of note, PFS and OS rates were similar regardless of MRD status after transplantation, showing that continuing with consolidation therapy mitigated the negative impact of suboptimal response for those patients who were still MRD-positive after ASCT.
Figure 2. Rates of 3-year OS and 3-year PFS according to the number of high-risk cytogenetic abnormalities*

OS, overall survival; PFS, progression-free survival.
*Adapted from Costa.1
- After a median follow up of 32.7 months from treatment cessation, the rates of 3-year OS and 3-year PFS according to the number of high-risk cytogenetic abnormalities in 84 patients who achieved an MRD-negative treatment-free state (MRD-SURE) are shown in Figure 3.
Figure 3. Rates of 3-year OS and 3-year PFS according to the number of high-risk cytogenetic abnormalities in patients who achieved an MRD-negative treatment-free state*

MRD, measurable residual disease; OS, overall survival; PFS, progression-free survival.
*Adapted from Costa.1
In patients reaching MRD-SURE status (N = 84):
- The 2-year risk of cumulative risk of progression is as follows:
- Zero high-risk cytogenetic abnormalities, standard risk was 9%
- One high-risk cytogenetic abnormality, high risk was 9%
- ≥2 high-risk cytogenetic abnormalities, ultra-high risk was 47%
- At the end of the study, 61 patients were still treatment free with sustained MRD-negativity
- The median follow-up after cessation of therapy was 32.7 months
- A total of 23 patients resumed treatment:
- 16 were due to disease progression
- 7 were due to MRD resurgence without progression
Safety
The adverse events reported in this final analysis did not differ much from previous reports, with 74% of patients experiencing an adverse event of Grade 3 or greater (for further details, see here). Additionally:
- A total of 25 serious treatment-emergent adverse events were recorded:
- Pneumonia, n = 8
- Pulmonary embolism, n = 3
- Fever and neutropenia, n = 2
- Atypical hemolytic uremic syndrome, n = 1
- Infusion-related reactions, n = 1
- Atrial fibrillation, n = 1
- Other, n = 9
- There were three treatment-emergent deaths:
- Two were unwitnessed
- One was due to pneumonia after transplantation
Conclusion
The final results from the MASTER trial show durable clinical responses for quadruplet induction therapy, with most patients who achieved treatment cessation having standard or high-risk cytogenetics. Moreover, around 80% of patients with either zero or one high-risk cytogenetic abnormality achieved MRD negativity. In contrast, achieving a sustained MRD-negative status in patients with ultra-high-risk cytogenetics remains an important unmet need. Future directions should now include new agents and mechanisms of action directed towards patients with persistent MRD as well as exploring the possibility of deferring ASCT in early MRD-negative patients.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



